Tabelle von Löslichkeitsprodukten - Teil 2
Autor: Hans Lohninger
Die folgende Tabelle listet die Löslichkeitsprodukte der wichtigsten, in Wasser schwerlöslichen, anorganischen Verbindungen auf. Werden in der Literatur verschiedene Löslichkeitsprodukte genannt, so sind diese mit Strichpunkten getrennt angeführt. Die Löslichkeitsprodukte von HgS und CuS sind so niedrig, dass sie nur schlecht experimentell bestimmt werden können.
| Substanz |
Formel |
Temperatur |
Ksp |
| Eisen(II)carbonat | FeCO3 | 18°C-25°C | 2×10-11 |
| | | 25°C | 3.13×10-11 |
| Eisen(II)fluorid | FeF2 | 25°C | 2.36×10-6 |
| Eisen(II)hydroxid | Fe(OH)2 | 18°C | 1.64×10-14 |
| | | 25°C | 1×10-15; 8.0×10-16; 4.87×10-17 |
| Eisen(II)oxalat | FeC2O4 | 25°C | 2.1×10-7 |
| Eisen(II)phosphat, Octahydrat | Fe3(PO4)2 8 H2O 8 H2O | 25°C | 1.7×10-29 |
| Eisen(II)sulfid | FeS | 18°C | 3.7×10-19 |
| | | 25°C | 8×10-19 |
| Eisen(III)hydroxid | Fe(OH)3 | 18°C | 1.1×10-36 |
| | | 25°C | 2.79×10-39 |
| Eisen(III)phosphat | FePO4 | 25°C | 1.3×10-22 |
| -, Dihydrat | FePO4 2 H2O 2 H2O | 25°C | 9.91×10-16 |
| Europium(III)hydroxid | Eu(OH)3 | 25°C | 9.38×10-27 |
| Gallium(III)hydroxid | Ga(OH)3 | 25°C | 7.28×10-36 |
| Kaliumhexachloroplatinat | K2PtCl6 | 25°C | 7.48×10-6 |
| Kaliumhydrogentartrat | KHC4H4O6 | 18°C | 3.8×10-4 |
| Kaliumperchlorat | KClO4 | 25°C | 1.05×10-2 |
| Kaliumperiodat | KIO4 | 25° | 3.71×10-4 |
| Kupfer(I)bromid | CuBr | 18°C-20°C | 4.15×10-8 |
| | | 25°C | 6.27×10-9 |
| Kupfer(I)chlorid | CuCl | 25°C | 1.72×10-7 |
| | | 18°C-20°C | 1.02×10-6 |
| Kupfer(I)cyanid | CuCN | 25°C | 3.47×10-20 |
Kupfer(I)hydroxid
(im Gleichgew. mit Cu2O + H2O) | CuOH | 25°C | 2×10-15 |
| Kupfer(I)iodid | CuI | 18°C-20°C | 5.06×10-12 |
| | | 25°C | 1.27×10-12 |
| Kupfer(I)sulfid | Cu2S | 16°C-18°C | 2×10-47 |
| Kupfer(I)thiocyanat | CuSCN | 18°C | 1.64×10-11 |
| | | 25°C | 1.77×10-13 |
| Kupfer(II)arsenat | Cu3(AsO4)2 | 25°C | 7.95×10-36 |
| Kupfer(II)carbonat | CuCO3 | 25°C | 1×10-10 |
| Kupfer(II)hydroxid | Cu(OH)2 | 18°C-25°C | 6×10-20 |
| | | 25°C | 4.8×10-20 |
| Kupfer(II)iodat | Cu(IO3)2 | 25°C | 1.4×10-7 |
| -, Monohydrat | Cu(IO3)2 H2O H2O | 25°C | 6.94×10-8 |
| Kupfer(II)oxalat | CuC2O4 | 25°C | 2.87×10-8, 4.43×10-10 |
| Kupfer(II)phosphat | Cu3(PO4)2 | 25°C | 1.40×10-37 |
| Kupfer(II)sulfid | CuS | 18°C | 8.5×10-45 |
| | | 25°C | 8×10-37 |
| Lanthanumiodat | La(IO3)3 | 25°C | 7.50×10-12 |
| Lithiumcarbonat | Li2CO3 | 25°C | 1.7×10-3; 8.15×10-4 |
| Lithiumfluorid | LiF | 25°C | 1.84×10-3 |
| Lithiumphosphat | Li3PO4 | 25° | 2.37×10-4 |
| Magnesiumammoniumphosphat | MgNH4PO4 | 25°C | 2.5×10-13; 3×10-13 |
| Magnesiumcarbonat | MgCO3 | 12°C | 2.6×10-5 |
| | | 25°C | 6.82×10-6 |
| -, Trihydrat | MgCO3 3 H2O 3 H2O | 25°C | 2.38×10-6 |
| -, Pentahydrat | MgCO3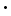 5 H2O 5 H2O | 25°C | 3.79×10-6 |
| Magnesiumfluorid | MgF2 | 18°C | 7.1×10-9 |
| | | 25°C | 5.16×10-11; 6.4×10-9 |
| Magnesiumhydroxid | Mg(OH)2 | 18°C | 1.2×10-11 |
| | | 25°C | 5.61×10-12 |
| Magnesiumoxalat | MgC2O4 | 18°C | 8.57×10-5 |
| Magnesiumoxalat, Dihydrat | MgC2O4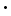 2 H2O 2 H2O | 25°C | 4.83×10-6 |
| Magnesiumphosphat | Mg3(PO4)2 | 25°C | 1.04×10-24 |
| Mangancarbonat | MnCO3 | 18°C-25°C | 9×10-11 |
| | | 25°C | 2.24×10-11 |
| Manganiodat | Mn(IO3)2 | 25°C | 4.37×10-7 |
| Manganhydroxid | Mn(OH)2 | 18°C | 4×10-14 |
| | | 25°C | 2×10-13 |
| Manganoxalat, Dihydrat | MnC2O4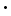 2 H2O 2 H2O | 25°C | 1.70×10-7 |
| Mangansulfid (rosa) | MnS | 18°C | 1.4×10-15 |
| | | 25°C | 3×10-11 |
| Mangansulfid (grün) | MnS | 25°C | 10-22; 3×10-14 |
| Neodymiumcarbonat | Nd2(CO3)3 | 25°C | 1.08×10-33 |
| Nickelcarbonat | NiCO3 | 25°C | 1.42×10-7 |
| Nickelhydroxid | Ni(OH)2 | 25°C | 5.48×10-16 |
| Nickeliodat | Ni(IO3)2 | 25°C | 4.71×10-5 |
| Nickelphosphat | Ni3(PO4)2 | 25°C | 4.74×10-32 |
| Nickelsulfid (alpha) | NiS | 25°C | 4×10-20 |
| | | 18°C-25°C | 10-21 |
| Nickelsulfid (beta) | NiS | 25°C | 1.3×10-25 |
| | | 18°C-25°C | 10-27 |
| Palladiumthiocyanat | Pd(SCN)2 | 25°C | 4.39×10-23 |
| Praseodymiumhydroxid | Pr(OH)3 | 25°C | 3.39×10-24 |
| Quecksilber(I)bromid | HgBr | 25°C | 1.3×10-21; 6.40×10-23 |
| Quecksilber(I)carbonat | Hg2CO3 | 25°C | 3.6×10-17 |
| Quecksilber(I)chlorid | Hg2Cl2 | 25°C | 2×10-18; 1.43×10-18 |
| Quecksilber(I)fluorid | Hg2F2 | 25°C | 3.10×10-6 |
| Quecksilber(I)iodid | HgI | 25°C | 1.2×10-28; 5.2×10-29 |
| Quecksilber(I)oxalat | Hg2C2O4 | 25°C | 1.75×10-13 |
| Quecksilber(I)sulfat | Hg2SO4 | 25°C | 6×10-7; 6.5×10-7 |
| Quecksilber(I)thiocyanat | Hg2(SCN)2 | 25°C | 3.2×10-20 |
| Quecksilber(II)bromid | HgBr2 | 25°C | 8×10-20; 6.2×10-20 |
| Quecksilber(II)chlorid | HgCl2 | 25°C | 2.6×10-15 |
Quecksilber(II)hydroxid
(im Gleichgew. mit HgO + H2O) | Hg(OH)2 | 25°C | 3.6×10-26 |
| Quecksilber(II)iodid | HgI2 | 25°C | 3.2×10-29; 2.9×10-29 |
| Quecksilber(II)sulfid | HgS | 18°C | 4×10-53 bis 2×10-49 |
| | | 25°C | 2×10-53; 2×10-54 |
|


 Anhang
Anhang  Tabellen
Tabellen  Löslichkeitsprodukte
Löslichkeitsprodukte  Löslichkeitsprodukte - Teil 2
Löslichkeitsprodukte - Teil 2





 Anhang
Anhang  Tabellen
Tabellen  Löslichkeitsprodukte
Löslichkeitsprodukte  Löslichkeitsprodukte - Teil 2
Löslichkeitsprodukte - Teil 2

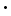 8 H2O
8 H2O