Standardbildungsenthalpie, freie Energie, und Standardentropie ausgewählter Substanzen
Autor: Hans Lohninger
Diese Tabelle führt die Standardbildungsenthalpie (ΔH°) und die freie Bildungsenergie (ΔG°) für die Bildung von Substanzen aus den Elementen im Normalzustand, und die thermodynamischen Entropien (S°) dieser Verbindungen bei 298 K auf.
Der Aggregatzustand der Substanzen wird durch folgende Symbole gekennzeichnet:
(g) .... gasförmig
(l) .... flüssig
(s) .... fest
(aq) .... wässrige Lösung
| Substanz |
ΔH0
[kJ mol-1] |
ΔG0
[kJ mol-1] |
S0
[J mol-1 K-1] |
| (COOH)2 (aq) | -818.3 | -697.9 | - |
| (COOH)2 (s) | -826.8 | -697.9 | 120 |
| (NH4)2SO4 (s) | -1179.3 | -900.35 | 220.3 |
| Ag (g) | 289.2 | 250.4 | 172.892 |
| Ag (s) | 0.0 | 0.0 | 42.702 |
| AgCl (s) | -127.03 | -109.72 | 96.11 |
| AgNO2 (s) | -44.371 | 19.85 | 128.1 |
| AgNO3 (s) | -123.1 | -37.2 | 140.9 |
| Al (g) | 314 | 273 | 164.44 |
| Al (s) | 0.0 | 0.0 | 28.32 |
| Al2O3 (s) | -1669.8 | -1576.4 | 50.986 |
| Al3+ (aq) | -524.7 | -481.2 | -313 |
| AlCl3 (s) | -653.4 | -636.8 | 167 |
| Ar (g) | 0.0 | 0.0 | 154.7 |
| As (g) | 253.7 | 212.3 | 174.1 |
| As (s, grau, metallisch) | 0.0 | 0.0 | 35 |
| As4 (s) | 149 | 105 | 289 |
| B (g) | 406 | 363 | 153.34 |
| B (s) | 0.0 | 0.0 | 6.53 |
| B2H6 (g) | 31 | 82.8 | 232.9 |
| B2O3 (s) | -1264 | -1184 | 54.02 |
| B5H9 (g) | 62.8 | 166 | 275.6 |
| Ba (g) | 175.6 | 144.8 | 170.28 |
| Ba (s) | 0.0 | 0.0 | 67 |
| Ba2+ (aq) | -538.36 | -560.7 | 13 |
BaCl2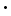 2H2O (s) 2H2O (s) | -1461.7 | -1296 | 203 |
BaCl2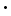 H2O (s) H2O (s) | -1165 | -1059 | 167 |
| BaCl2 (s) | -860.06 | -810.9 | 126 |
| BBr3 (g) | -187 | -213 | 324.2 |
| BBr3 (l) | -221 | -219 | 229 |
| BCl3 (g) | -395 | -380 | 289.9 |
| BCl3 (l) | -418.4 | -379 | 209 |
| Be (g) | 320.6 | 282.8 | 136.17 |
| Be (s) | 0.0 | 0.0 | 9.54 |
| Be2+ (aq) | -390 | -356 | - |
| BF3 (g) | -1110 | -1093 | 254.0 |
| BF4 (aq) | -1527 | -1435 | 167 |
| Br (g) | 111.8 | 82.38 | 174.913 |
| Br- (aq) | -120.9 | -102.82 | 80.71 |
| Br2 (g) | 30.7 | 3.14 | 245.35 |
| Br2 (l) | 0.0 | 0.0 | 152.3 |
| C (g) | 718.384 | 672.975 | 157.99 |
| C (s, Diamant) | 1.896 | 2.866 | 2.439 |
| C (s, Graphit) | 0.0 | 0.0 | 5.694 |
| C2H2 (g) | 226.75 | 209 | 200.82 |
| C2H4 (g) | 52.283 | 68.124 | 219.5 |
| C2H5OH (g) | -235.4 | -168.6 | 282 |
| C2H5OH (l) | -277.63 | -174.8 | 161 |
| C2H6 (g) | -84.667 | -32.88 | 229.5 |
| C2O42- (aq) | -824.2 | -674.9 | 51.0 |
| C3H8 (g) | -103.8 | -23.5 | 269.9 |
| C6H12O6 (s) | -1260 | -919.2 | 288.9 |
| C6H6 (g) | 82.927 | 129.65 | 269.2 |
| C6H6 (l) | 49.028 | 124.50 | 172.8 |
| Ca (g) | 192.6 | 158.9 | 154.8 |
| Ca (s) | 0.0 | 0.0 | 41.6 |
| Ca2+ (aq) | -542.96 | -553.04 | -55.2 |
| CaCO3(s, Aragonit) | -1207.0 | -1127.7 | 88.7 |
| CaCO3(s, Calcit) | -1206.9 | -1128.8 | 92.9 |
| CH3CHO (aq) | -208.7 | - | - |
| CH3CHO (g) | -166.4 | -133.7 | 266 |
| CH3CHO (l) | -195 | - | - |
| CH3COO- (aq) | -488.871 | -372.5 | - |
| CH3COOH (aq) | -488.453 | -399.6 | - |
| CH3COOH (l) | -487.0 | -392 | 160 |
| CH3NH2 (g) | -28 | 28 | 241.5 |
| CH3OCH3 (g) | -184.05 | -112.92 | 267.1 |
| CH3OH (aq) | -245.9 | -175.2 | 132.3 |
| CH3OH (g) | -201.3 | -161.9 | 236 |
| CH3OH (l) | -238.64 | -166.3 | 127 |
| CH3SH (g) | -12.4 | 0.88 | 254.8 |
| CH4 (g) | -74.848 | -50.794 | 186.2 |
| Cl (g) | 121.39 | 105.40 | 165.09 |
| Cl- (aq) | -167.46 | -131.17 | 55.2 |
| Cl2 (g) | 0.0 | 0.0 | 222.95 |
| Cl2O (g) | 76.15 | 93.72 | 266.5 |
| ClF3 (g) | -155 | -114 | 278.7 |
| ClO- (aq) | - | -37 | 43.1 |
| ClO2 (g) | 103 | 123 | 249 |
| ClO2- (aq) | -69.0 | -10.7 | 101 |
| ClO3- (aq) | -98.32 | -2.6 | 163 |
| ClO4- (aq) | -131.4 | -8 | 182 |
| CO (g) | -110.523 | -137.268 | 197.91 |
| CO2 (aq) | -412.9 | -386.2 | 121 |
| CO2 (s) | -393.513 | -394.383 | 213.64 |
| CO32- (aq) | -676.3 | -528.1 | -53.1 |
| Cs (g) | 78.78 | 51.21 | 175.49 |
| Cs (s) | 0.0 | 0.0 | 82.8 |
| Cs+ (aq) | -248 | -282.0 | 133 |
| CsBr (s) | -394 | -383 | 121 |
| CsCl (s) | -433.0 | -404.1 | - |
| CsF (s) | -530.9 | -500.0 | - |
| CsI (s) | -337 | -333 | 130 |
| Cu (g) | 341.0 | 301.4 | 166.28 |
| Cu (s) | 0.0 | 0.0 | 33.3 |
| Cu+ (aq) | 51.9 | 50.2 | -26 |
| Cu2+ (aq) | 64.39 | 64.98 | -98.7 |
| CuSO4 (s) | -769.86 | -661.9 | 113 |
| F (g) | 76.6 | 59.4 | 158.64 |
| F- (aq) | -329.1 | -276.4 | -9.6 |
| F2 (g) | 0.0 | 0.0 | 203 |
| Fe (g) | 404.5 | 358.8 | 100.3 |
| Fe (s) | 0.0 | 0.0 | 27.2 |
| Fe2+ (aq) | -87.9 | -84.93 | -113 |
| Fe2O3(s, Hämatit) | -822.1 | -741.0 | 90.0 |
| Fe3+ (aq) | -47.7 | -10.6 | -293 |
| Fe3O4(s, Magnetit) | -1121 | -1014 | 146 |
| Ge (g) | 328.2 | 290.8 | 167.8 |
| Ge (s) | 0.0 | 0.0 | 42.42 |
| H (g) | 217.94 | 203.24 | 114.61 |
| H+ (aq) | 0.0 | 0.0 | 0.0 |
| H2 (g) | 0.0 | 0.0 | 130.59 |
| H2CO3 (aq) | -698.7 | -623.4 | 191 |
| H2O (g) | -241.83 | -228.59 | 188.72 |
| H2O (l) | -285.84 | -237.19 | 69.940 |
| H2O2 (aq) | -191.1 | -131.67 | - |
| H2O2 (l) | -187.6 | -113.97 | 92 |
| H2S (aq) | -39 | -27.4 | 122 |
| H2S (g) | -20.15 | -33.02 | 205.6 |
| H30+(aq) | -285.84 | -237.19 | 69.940 |
| HBr (g) | -36.2 | -53.22 | 198.48 |
| HC2O4- (aq) | -818.8 | -698.7 | 154 |
| HCHO (aq) | - | -130 | - |
| HCHO (g) | -116 | -110 | 218.7 |
| HCl (aq) | -167.46 | -131.17 | 55.2 |
| HCl (g) | -92.312 | -95.265 | 186.68 |
| HClO (aq) | -116.4 | -79.956 | 130 |
| HCO3 (aq) | -691.1 | -587.1 | 95.0 |
| HCOO- (aq) | -410.0 | -334.7 | 91.6 |
| HCOOH (aq) | -410.0 | -356.1 | 164 |
| HCOOH (g) | -362.6 | -335.7 | 251 |
| HCOOH (l) | -409.2 | -346.0 | 129.0 |
| He (g) | 0.0 | 0.0 | 126.1 |
| HF (g) | -269 | -271 | 173.5 |
| Hg (g) | 60.84 | 31.8 | 174.9 |
| Hg (l) | 0.0 | 0.0 | 77.4 |
| Hg2Cl2 (s) | -264.9 | -210.7 | 196 |
| HgCl2 (s) | -230 | -186 | 144 |
| HI (g) | 26 | 1.3 | 206.32 |
| I (g) | 106.61 | 70.149 | 180.68 |
| I- (aq) | -55.94 | -51.67 | 109.4 |
| i-C4H10 (g) | -131.6 | -18.0 | 294.6 |
| I2 (aq) | 21 | 16.43 | - |
| I2 (g) | 62.241 | 19.4 | 260.58 |
| I2 (s) | 0.0 | 0.0 | 117 |
| I3- (aq) | -51.9 | -51.51 | 174 |
| IBr (g) | 40.8 | 3.8 | 259 |
| ICl (g) | 18 | -5.52 | 247.4 |
| ICl3 (s) | -88.3 | -22.6 | 172 |
| K (g) | 90.00 | 61.17 | 160.23 |
| K (s) | 0.0 | 0.0 | 63.6 |
| K+ (aq) | -251.2 | -282.2 | 103 |
| KCl (g) | -219 | -235 | 239.5 |
| KCl (s) | -435.87 | -408.32 | 82.68 |
| Kr (g) | 0.0 | 0.0 | 164.0 |
| Li (g) | 155.1 | 122.1 | 138.67 |
| Li (s) | 0.0 | 0.0 | 28.0 |
| Li+ (aq) | -278.46 | -293.8 | 14 |
| LiBr (s) | -350.3 | -339.9 | 69.0 |
| LiCl (s) | -408.8 | -383.6 | 55.2 |
| LiF (s) | -612.1 | -584.1 | 35.9 |
| LiI (s) | -271.1 | -268 | - |
| Mg (g) | 150 | 115 | 148.55 |
| Mg (s) | 0.0 | 0.0 | 32.5 |
| Mg2+ (aq) | -461.96 | -456.01 | -118 |
| MgCl2-6 H2O (s) | -2499.6 | -2115.6 | 366 |
| MgCl2 (s) | -641.83 | -542.32 | 89.5 |
| N (g) | 472.646 | 455.512 | 153.195 |
| n-C4H10 (g) | -124.7 | -15.7 | 310.0 |
| n-C8H18 (l) | -250.0 | 6.48 | 360.8 |
| N2 (g) | 0.0 | 0.0 | 191.49 |
| N2H4 (l) | 50.42 | - | - |
| N2O (g) | 81.55 | 103.6 | 220.0 |
| N2O22- (aq) | -10.8 | 138 | 28 |
| N2O4 (g) | 9.661 | 98.286 | 304.3 |
| N2O5 (s) | -41:8 | 134 | 113 |
| N3- (aq) | 252 | 325 | 134 |
| Na (g) | 108.7 | 71.11 | 153.62 |
| Na (s) | 0.0 | 0.0 | 51.0 |
| Na+ (aq) | -239.66 | -261.87 | 60.2 |
| Na2 (g) | 142.1 | 104.0 | 230.2 |
| Na2CO3 (s) | -1131 | -1048 | 136 |
| Na2O (s) | -416 | -377 | 72.8 |
| Na2O2 (s) | -504.6 | -430.1 | 66.9 |
| NaBr (s) | -359.95 | -347.7 | - |
| NaCl (s) | -411.00 | -384.03 | 72.4 |
| NaF (s) | -569.0 | -541.0 | 58.6 |
| NaI (s) | -288.0 | -237 | - |
| NaO2 (s) | -259 | -195 | - |
| Ne (g) | 0.0 | 0.0 | 144.1 |
| NH2CONH2 (s) | -333.2 | -197.2 | 104.6 |
| NH3 (aq) | -80.83 | -26.6 | 110 |
| NH3 (g) | -46.19 | -16.64 | 192.5 |
| NH4+ (aq) | -132.8 | -79.50 | 112.8 |
| NH4Cl (s) | -315.4 | -203.9 | 94.6 |
| NO (g) | 90.374 | 86.688 | 210.62 |
| NO2- (aq) | -106 | -34.5 | 125 |
| NO2 (g) | 33.85 | 51.840 | 240.5 |
| NO3- (aq) | -206.57 | -110.6 | 146 |
| O (g) | 247.52 | 230.09 | 160.95 |
| O2 (g) | 0.0 | 0.0 | 205.03 |
| O3 (g) | 142 | 163.4 | 238 |
| OH (g) | 42.09 | 37.4 | 183.63 |
| OH- (aq) | -229.94 | -157.30 | -10.5 |
| P (g) | 314.5 | 279.1 | 163.1 |
| P (s, rot) | -18 | -14 | 29 |
| P (s, weiß) | 0.0 | 0.0 | 44.4 |
| P4 (g) | 54.89 | 24.4 | 279.9 |
| Pb (g) | 193.9 | 161.0 | 175.27 |
| Pb (s) | 0.0 | 0.0 | 64.89 |
| Pb2+ (aq) | 1.6 | -24.3 | 21 |
| PbO (s, rot) | -219.2 | -189.3 | 67.8 |
| PbO (s, gelb) | -217.9 | -188.5 | 69.5 |
| PbO2 (s) | -276.6 | -219.0 | 76.6 |
| PCl3 (g) | -306.4 | -286.3 | 311.7 |
| PCl5 (g) | -398.9 | -324.6 | 353 |
| Rb (g) | 85.81 | 55.86 | 169.99 |
| Rb (s) | 0.0 | 0.0 | 69.5 |
| Rb+ (aq) | -246 | -282.2 | 125 |
| RbBr (s) | -389.2 | -378.1 | 108.3 |
| RbCl (s) | -430.58 | -405 | - |
| RbF (s) | -551.9 | -520.0 | 114 |
| RbI (s) | -328 | -326 | 118.0 |
| Rn (g) | 0.0 | 0.0 | 176.1 |
| S (g) | 222.8 | 182.3 | 167.72 |
| S (s, monoklin) | 0.30 | 0.096 | 32.6 |
| S (s, rhombisch) | 0.0 | 0.0 | 31.9 |
| SO2 (g) | -296.9 | -300.4 | 248.5 |
| SO3 (g) | -395.2 | -370.4 | 256.2 |
| S2- (aq) | 41.8 | 83.7 | - |
| Si (g) | 368.4 | 323.9 | 167.86 |
| Si (s) | 0.0 | 0.0 | 18.7 |
| SiO (g) | -111.8 | -137.1 | 206.1 |
| SiO2 (s, Quarz) | -859.4 | -805.0 | 41.84 |
| Sn (g) | 300 | 268 | 168.4 |
| Sn (s, grau) | 2.5 | 4.6 | 44.8 |
| Sn (s, weiß) | 0.0 | 0.0 | 51.5 |
| SO (g) | 79.58 | 53.47 | 221.9 |
| Sr (g) | 164 | 110 | 164.53 |
| Sr (s) | 0.0 | 0.0 | 54.4 |
| Sr2+ (aq) | -545.51 | -557.3 | -39 |
| Ti (g) | 469 | 423 | 100.20 |
| Ti (s) | 0.0 | 0.0 | 30.3 |
| Ti2O3 (s) | -1536 | -1448 | 78.78 |
| Ti3O5 (s) | -2443 | -2300 | 129.4 |
| TiO2 (s, Rutil III) | -912.1 | -852.7 | 50.25 |
| TiO2+ (aq) | - | -577 | - |
| TlI (s) | -124 | -124 | 123 |
| TlI (g) | 33 | -13 | 274 |
| W (g) | 843.5 | 801.7 | 173.85 |
| W (s) | 0.0 | 0.0 | 33 |
| Xe (g) | 0.0 | 0.0 | 169.6 |
| Zn (s) | 0.0 | 0.0 | 41.6 |
| ZnO (s) | -348.0 | -318.2 | 43.9 |
|


 Anhang
Anhang  Tabellen
Tabellen  Standardbildungsenthalpie, freie Energie, und Standardentropie ausgewählter Substanzen
Standardbildungsenthalpie, freie Energie, und Standardentropie ausgewählter Substanzen





 Anhang
Anhang  Tabellen
Tabellen  Standardbildungsenthalpie, freie Energie, und Standardentropie ausgewählter Substanzen
Standardbildungsenthalpie, freie Energie, und Standardentropie ausgewählter Substanzen

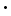 2H2O (s)
2H2O (s)